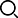Biological umbilical cord protection
Our company is a modern enterprise specializing in the research and development, manufacturing and sales of medical consumables. The company is located in Changshan Industrial Park, Jinxian County, Nanchang City, Jiangxi Province, within the half-hour economic circle of Nanchang City, adjacent to the Shanghai-Kunming Expressway, Fuyin Expressway, Liwen Expressway, and National Highway 320, with very convenient transportation. It is very suitable for the entrepreneurship and development of enterprises.
keywords
Biological umbilical cord protection
- DESCRIPTION
-
Instructions for use of biological umbilical cord protection products
Warning: This product is sterile and should be used once. It is prohibited to use if the packaging is damaged or expired.
[Product name] Biological umbilical cord protection
[Manufacturer license number] Gan Food and Drug Administration Machinery Production License No. 20160098
[Product registration certificate number] Gan Machinery Registration No. 20152140268
[Product technical requirements number] Gan Machinery Registration No. 20152140268
[Main structure of the product] The product consists of three parts: outer belt, core and accessories. The outer belt is made of loop velvet, knitted cotton cloth and nylon buckle by sewing; the core is a small bag made of high molecular absorbent resin and bamboo charcoal wrapped in heat-sealed tea packaging paper, wrapped in chitin non-woven fabric or knitted fabric; the accessories are made of latex tubes (umbilical rings) pierced by non-absorbent surgical sutures, cotton swabs, and gauze blocks, which are sealed and packaged.
[Specifications and models] Umbilical cord protection is divided into two specifications according to the shape of the inner core: type A (round) and type B (elliptical).
[Product Performance] This product can stop bleeding by tying the umbilical cord, keep the umbilical cord dry, accelerate the natural shedding of the umbilical cord, prevent umbilical infection, and promote umbilical healing. It also accelerates the discharge of meconium in newborns and reduces the incidence of neonatal jaundice.
[Scope of Application] It is used by medical institutions and families for the prevention and care of umbilical infection in newborns.
[Instructions] After the umbilical cord of the newborn is cut and treated routinely, the inner pad of this product is applied to the umbilical cord of the newborn and fixed. Change it every 24 hours. The course of treatment depends on the individual situation of the newborn, generally 6-8 days, until the umbilical cord heals.
[Precautions]
1. The umbilical cord is only for professional medical staff to operate and use. If the umbilical cord is broken, it should not be used.
2. It should be replaced in time when wet or contaminated.
3. When using and replacing, attention should be paid to aseptic operation and umbilical disinfection. It is recommended to be carried out under the guidance of medical staff.
4. This product is sterile and should be used once.
5. When used for umbilical infection, it should be combined with other treatments.
6. If there are very few special physical infants with abnormal reactions such as local skin allergies, the product should be discontinued immediately.
[Contraindications] Do not use if the packaging is damaged or expired.
[Sterilization method and validity period] Sterilized by cobalt-60 irradiation, the sterilization validity period is 30 months.
Inquiry
Attention: Please leave your contact information and email, and our professionals will contact you as soon as possible!