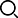Absorbent gelatin sponge regular type 20*20*5mm
This product is suitable for hemostasis in wound bleeding areas, emergency hemostasis and surgical hemostasis.
keywords
Absorbent gelatin sponge regular type 20*20*5mm
- DESCRIPTION
-
[Product Name] Absorbent gelatin sponge
[Main structure and performance] This product uses medicinal gelatin derived from pig skin as raw material, and is made through foaming, curing, drying, sterilization and other processes.
[Main ingredients] This product is a collagen product with a protein content of more than 85%.
[Scope of application] This product is used for hemostasis of wound bleeding areas, emergency hemostasis and surgical hemostasis.
[Instructions for use] Before use, the product packaging must not be damaged, damp or contaminated, and the wound surface must be cleaned and disinfected. The process of removing the product from the package should be carried out under sterile conditions; only a low amount is used for hemostasis, and excess gelatin sponge should be removed by moistening the excess product around the bleeding site with sterile saline, then removing it, and retaining the sponge adhering to the bleeding site; this product can be used in the bleeding site for hemostasis using the "dry use method" and "saline infiltration method".
[Precautions] 1. A small amount of this product should be used to achieve the purpose of hemostasis. Once the bleeding is stopped, the excess product should be removed or discarded to facilitate absorption and reduce foreign body reactions. 2. When this product is placed in a cavity or closed tissue space, it is recommended to apply light pressure in advance to avoid excessive packing. The product may expand to its original size after absorbing body fluids, compressing the surrounding nerves and causing damage to them. 3. Sometimes hemostatic surgery requires filling the cavity, but the excess product other than the amount used to maintain the hemostatic effect should be removed. 4. This product cannot be used with methyl methacrylate adhesive. Because collagen fibers may reduce the strength of the methyl methacrylate adhesive that bonds the repair device to the bone surface. 5. This product should not be used to treat diseases with primary coagulation disorders. 6. This product cannot be used simultaneously with autologous blood circulation rescue. 7. When this product is used in or around bone holes, bone fixation areas, spinal cord and/or optic nerves and optic chiasm, it should be removed after hemostasis. 8. This product cannot be used after using silver nitrate or any other corrosive chemicals, because the chemical burning area will affect the absorption of this product.
【Storage】 It should be stored in a dry (relative humidity does not exceed 80%) storage room with a temperature controlled at 15-30 0C (59-86 0F). It should not be mixed with toxic, harmful or odorous items.
【Production date】 See the label.
【Production batch number】 See the label.
【Expiration date】 See the label.
【Registrant/Manufacturer name】: Jiangxi Xiangen Medical Technology Development Co., Ltd.
【Registrant/Manufacturer address】: Beside Lichang Road, Changshan Township, Jinxian County, Nanchang City, Jiangxi Province
【Contact information】Tel: 0791-85602328 Fax: 0791-85602919
Inquiry
Attention: Please leave your contact information and email, and our professionals will contact you as soon as possible!